Biological Sciences celebrates its third AAAS fellow, Miriam Greenberg
Profe ssor Miriam Greenberg has been elected as a 2022 AAAS Fellow, one of the highest honors within the scientific community. Conferred by the American Association for the Advancement of Science (AAAS), which is the world’s largest general scientific society and publisher of the respected research journal Science, the AAAS fellowship recognizes members “whose efforts on behalf of the advancement of science or its applications are scientifically or socially distinguished.” Dr. Greenberg joins former chairs Dave Njus and Vicky Meller as the third AAAS fellow in the department.
ssor Miriam Greenberg has been elected as a 2022 AAAS Fellow, one of the highest honors within the scientific community. Conferred by the American Association for the Advancement of Science (AAAS), which is the world’s largest general scientific society and publisher of the respected research journal Science, the AAAS fellowship recognizes members “whose efforts on behalf of the advancement of science or its applications are scientifically or socially distinguished.” Dr. Greenberg joins former chairs Dave Njus and Vicky Meller as the third AAAS fellow in the department.
“It is nice to be acknowledged for what you’ve contributed,” remarked Greenberg. “It is an honor and I am very appreciative.” She quickly added that her accomplishments so far would not have been possible without the input of her lab members, including the many master’s program and doctoral students who have brought ideas, enthusiasm, and dedication to her WSU research over the past three decades. “We have really terrific graduate students at Wayne State, and I have been very fortunate to have them in my group,” she remarked.
Greenberg’s contributions to science are the outcome of dogged hard work, unending energy, keen scientific curiosity, openness to new ideas, and readiness to collaborate. This recipe has led to a collection of important breakthroughs in two areas. One centers on cardiolipin, an understudied molecule that plays a role in the confounding genetic disorder called Barth syndrome; and the other focuses on inositol, a type of sugar, and provides insight into why commonly prescribed bipolar disorder drugs work. In the long term, these advances may help guide the development of new and better drugs.
Many of these findings came from ideas generated by the students and their work,” she remarked. “I have been extremely lucky to have had such wonderful students all along the way. I would not have had a research program without them, that’s for sure.
Keys to Barth Syndrome
Greenberg’s studies of cardiolipin began when she first established her own research lab. “Cardiolipin is present in all eukaryotes — from yeast to humans — and wouldn’t have been saved over evolutionary time if it wasn’t doing something important. So, it seemed like a good area to pursue,” she recalled.
Her research took on added significance when Barth syndrome was found to result from mutations in cardiolipin metabolism. “Suddenly, cardiolipin had a connection to a genetic disorder,” she said. That led to meetings and collaborations with pediatric neurologist Peter Barth, who identified the syndrome, and the active Barth research group of Ronald J. A. Wanders at the University of Amsterdam, as well as an introduction to the newly formed Barth Syndrome Foundation, for which Greenberg now serves as a member of the scientific and medical advisory board and board of directors.
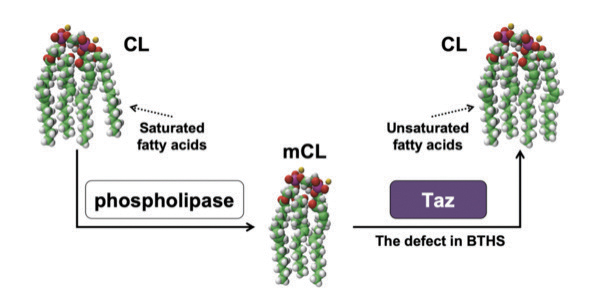
Over the years, Greenberg’s group has made many important findings related to cardiolipin and its connection to Barth syndrome. A few highlights (and students involved) include:
- The development of the first model organism, a yeast model by Zhiming Gu (Ph.D. 2002), and then a second model in mouse myoblast cells by Wenjia Lou (Ph.D. 2017). “These have been very powerful models for us and for other researchers to study the function of the mutated gene, and analyze what’s going on in Barth syndrome,” Greenberg described.
- The discovery by graduate student Vaishnavi Raja (Ph.D. 2016) that cardiolipin-deficient yeast mutants have decreased synthesis of the molecule acetyl-CoA, which in turn influences the cellular-energy-producing machine known as the Krebs cycle. Subsequently, Yiran Li (Ph.D. 2018) and Zhuqing Liang (Ph.D. 2023) showed that this deficiency was also present in cardiolipin-deficient mouse cells. “I was blown away by this finding, because cardiolipin is a mitochondrial membrane lipid, while energy metabolism in the Krebs cycle takes place in the mitochondrial matrix (inside the organelle).”
- The determination by graduate student Linh Vo (Ph.D. 2023) that the mutated gene in Barth syndrome almost entirely shuts down the activity of a gene (MyoD1) that is important for muscle cell development, and therefore may provide insight into the muscle weakness that is one of the common symptoms of the disorder.
- The finding that a cardiolipin-enhanced enzyme (peroxidase) may be a primary cause of Barth syndrome. “If that’s the case, then we can try to identify inhibitors of this enzyme and potentially find an effective treatment,” she said. This result is the outcome of a collaborative study with Dr. Valerian Kagan at the University of Pittsburgh.
Insights into inositol
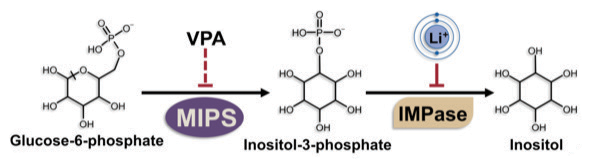
The inositol side of her research program grew from her graduate work in the Albert Einstein College of Medicine lab of lipid researcher Susan Henry, her doctoral advisor. “My goal was to use yeast as a model in order to identify genes that are involved in regulation of inositol synthesis,” she described. A few years after she earned her doctorate and right about the time she was immersing herself in cardiolipin studies, Greenberg saw a new research study indicating that lithium, one of the major drugs used to treat bipolar disorder, inhibited the synthesis of inositol. “I was sure researchers would be clamoring to follow up with additional yeast experiments,” she said, “but a year went by, then another, and nobody did.”
That obvious gap got the best of her, and she added inositol studies to her lab’s already full schedule. She described, “A major experiment that graduate students Deirdre Vaden (Ph.D. 2000) and Daobin Ding (2001) did early on was to see if another common drug (valproic acid) in the bipolar-disorder arsenal affects inositol synthesis, too, and we found that it did. And when two completely, structurally different drugs affect the same pathway, it suggests that pathway may be involved in the therapeutic mechanism, so that was a pretty major finding,” she said.
From there, Greenberg’s research group got to work determining how inositol synthesis is regulated in human cells. “It was quite a challenge, but graduate student Mahmoud Suliman (Ph.D. 2022) was not only able to make the first inositol mutant in a human cell line, but also carried out a very large analysis of what happens when you starve the human cells for inositol, and with the help of collaborators in Amsterdam, we found an eye-popping array of changes, including the upregulation of bioactive lipids called ceramides,” she said. Students Cunqi Ye (Ph.D. 2014), Wenxi Yu (Ph.D. 2016), Pablo Lazcano, and others also identified for the first time a gene (IP6K1) that regulates inositol synthesis in human cells.
With the help of a $1.95 million grant, the Greenberg lab is continuing its investigations. “We now know that at least three commonly prescribed bipolar drugs inhibit inositol synthesis, so we will be conducting a very rigorous study to look at the big picture of the cellular consequences.” She added, “That’s important because if regulating inositol synthesis turns out to be a major therapeutic mechanism, then understanding that mechanism can lead to the development of new, more effective drugs that work for everybody, but don’t have the side effects seen with current drugs, such as lithium and valproate.”
Power of students
In all of her lab’s research on inositol and cardiolipin, and behind the accomplishments that led to her election as AAAS fellow, Greenberg reiterated that the students have fueled the successes. “Many of these findings came from ideas generated by the students and their work,” she remarked. “I have been extremely lucky to have had such wonderful students all along the way. I would not have had a research program without them, that’s for sure.”