
Hanaa Hariri: Decoding the Mysterious Crosstalk Inside Cells
Eavesdropping on Organelles
Beneath the constant buzz of cell-to-cell communication that keeps living things humming along, another little-explored network is whispering back and forth. This network runs within each cell and between its internal machines: the organelles.
“For a long time, we thought that these organelles just float around the cell and work separately, but we now know they interact with one another and are physically connected by proteins that we call tethers. This has opened up a new area in biology with a whole set of questions: What are the tethers? What do they do? How do they function?” explained Hanaa Hariri, Ph.D., whose WSU lab is trying to solve some of the mysteries surrounding tethers and organelle communication.
“I find myself very drawn to challenges, and I was excited about the opportunity to make discoveries in this new area of research,” she said. Hariri and her lab are particularly interested in the roles that tethers and organelle communication play in metabolic pathways, or the complex series of enzyme-regulated reactions and interactions necessary to transform food to energy, and therefore to sustain life.
To study this topic, Hariri’s group is deviating from the traditional research approach of breaking cells apart and studying the metabolic enzymes in a test tube. “Advances in high-resolution microscopy and other techniques are now allowing us to see what happens in a living cell,” she said. The distinction is important, because the environment in a living cell is always changing, and that can greatly impact its function.
She explained why: Each reaction in a metabolic pathway requires specific enzymes as well as the substrates on which the enzymes act, so if either isn’t available near the reaction site, the reaction can’t run. If that happens, it can bring the metabolic pathway to a screeching halt. Hariri and her team are deciphering this complicated labyrinth of reactions and interactions with a variety of experiments.
This includes genetically modifying a cell so it cannot produce a certain enzyme, seeing how the cell changes its shape or organelle locations in response; and performing so-called “rescue experiments” where the researchers then supply the removed enzyme as a way to spot the exact reaction that is impacted. Other experiments involve adding an oversupply of lipids or other excess substrates to see how the cell responds or isolating a tether to observe which enzymes it recruits and why.

“Tethers are fascinating because they are proteins that have domains on one end that insert in one organelle’s membrane, and other domains that insert in another organelle’s membrane, so they form a bridge — a molecular bridge — to hold organelles together,” she described. “Those bridges can be dynamic or static, so they can form and expand under certain conditions, and also deform. In addition, some of the tethers have been shown to form connections between more than two organelles at a time.”
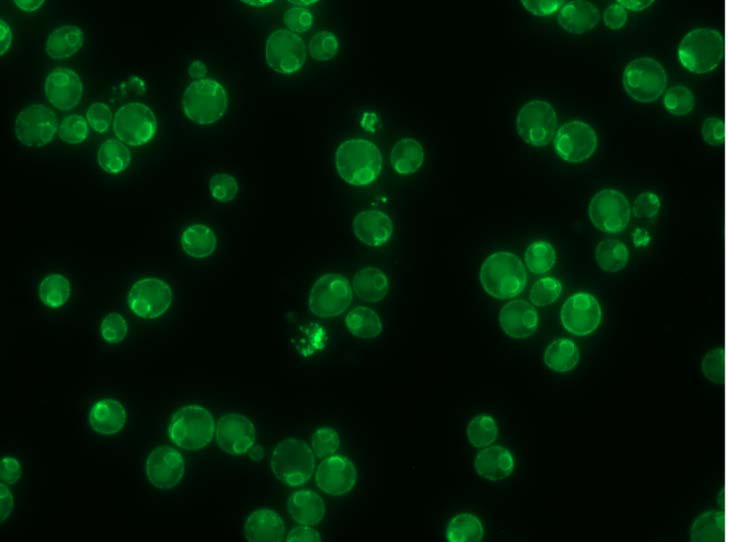
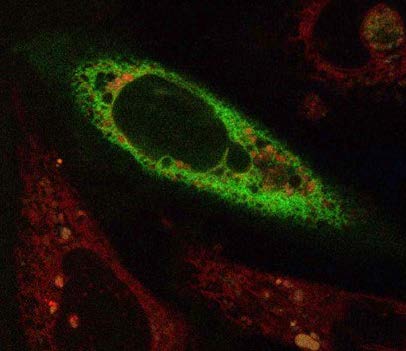
Although the reason for the bridge formation isn’t completely clear, Hariri believes it may help put the organelles in the best alignment for a needed task, an idea that builds on some of her earlier work. “During my postdoc, we proposed that the tether-contact sites between organelles can act as platforms that are able to recruit different enzymes to perform a specific function at certain times.
For instance, if a cell needs to either convert or store fatty acids at a certain site, the tether would have the capacity to bring in all the enzymes that are needed to do this function,” she said. “This is why spatial arrangement matters, and why my research group and I are now combining microscopy with other techniques, such as genetics, biochemistry and proteomics, to understand what is going on when everything is running smoothly, and also when it isn’t.”
Unlike many scientists who decide on research questions based on what their techniques can do, Hariri prefers to decide on the question first and figure out how to answer it later. “I think this is the perfect field to have this question-driven, big-picture mentality because it's very new and there are many unknowns,” she said. “And my lab members seem to like it, too.”
I think that learning to ask the right question is a much more transferable skill for my students than learning the mechanistic detail of one specific technique.
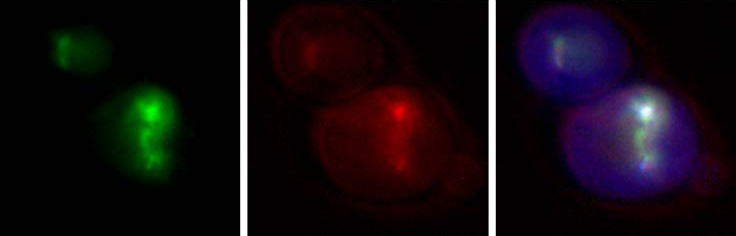
Her research group includes between six and seven graduate and undergraduate students, and a research assistant. “I think that learning to ask the right question is a much more transferable skill for my students than learning the mechanistic detail of one specific technique, and it also encourages a lot of conversations about which techniques and which collaborations might help us find answers. So, we ask a lot of questions and reach out to a lot of people.”

Her group’s research question at the moment, she said, is how tethers assist in the crosstalk between different metabolic networks, specifically between the networks involved in lipid metabolism and in amino acid metabolism. Glitches in this interplay could have implications for a variety of health conditions, including obesity, diabetes and fatty liver disease, so this work could ultimately help guide the development of treatments.
She added, “There’s nothing about biology or how nature works that is not going to be connected to human health somehow, and that’s why we need to look at the big picture, ask the right questions, and get that foundational understanding. There’s still so much to learn.”
Learn more about work in the Hariri laboratory.
Dr. Hanaa Hariri received her master’s in cell biology from the American University of Beirut and her doctorate in biophysics from Florida State University. She was a postdoctoral researcher at the University of Texas Southwestern Medical Center before joining Wayne State's biological sciences department in 2021.